Who is this post for
This post is an almost direct transcription of the first paragraphs of Chapter 1, of my PhD thesis. When I was writing it, I assumed the reader would have at least a basic knowledge of biology, and some familiarity with genetics and developmental biology. Some terms may be hard to digest if you’re not familiar with the science / history behind them. I hope this does not discourage you, but if it does, I provided a Glossary at the end of this page, to help guide you through it.
A brief definition of epigenetics
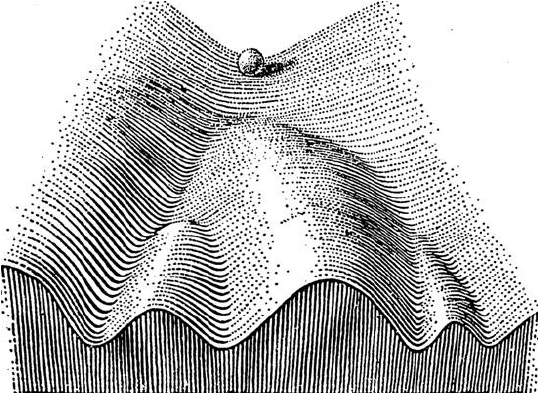
The term epigenetics was first coined by Conrad Waddington in 1948 (Waddington 2012) to define the interaction between cell differentiation (epigenesis) and genetic activity. Waddington described his ideas using the widely known metaphor of developmental epigenetic landscapes (Waddington 2014) (illustrated in the figure above). Take a look at this excerpt below, from The Strategy of the Genes:
“Part of an epigenetic landscape. The path followed by the ball, as it rolls down towards the spectator, corresponds to the developmental history of a specific cell in a ferilized embryo. There is first an alternative, towards the right or left. Along the former path, a second alternative is offered; along the path to the left, the main channel continues leftwards, but there is an alternative path which, however, can only be reached over a threshold.” Reproduced from “The strategy of the genes: a discussion of some aspects of theoretical biology”, by C.H. Waddington, 1957, under Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) license.”
Briefly, we can imagine some marbles standing on top of a hill which represent totipotent (or undifferentiated) cells at the beginning of embryogenesis. As time goes by, cells move downhill within channels (or “creodes”), with bifurcations representing differentiation outcomes, which will eventually lead to their differentiated state. As a cell moves downhill, the paths available become more limited. These paths represent a cell developmental lineage. On a further note, Waddington proposed that genetic mutations that alter the “creodes” down the slope would have an impact on cell differentiation. However, transcription regulators and their activity in relation to genes were never actually referred to by Waddington, when addressing epigenetics since the actual structure of DNA was only discovered about a decade later.
A few years later, John Gurdon et al. (Gurdon, Elsdale, and Fischberg 1958) transplanted single somatic nuclei from differentiated cells into an enucleated egg of Xaenopus laevis and observed these contained sufficient genetic information for the development of sexually mature frogs. Gurdon et al. were insightful enough to make two possible conclusions: either the transferred nuclei were undifferentiated and totipotent, or they were differentiated, but able to return to a totipotent state in the cytoplasm of an un-cleaved egg. The latter is now known to be true, and it supported Waddington’s idea that as a cell moves down the slope of the epigenetic landscape, its genetic activity is altered, limiting its potential to differentiate.
In mammals, aside from genetic variants, other factors such as genetically and non-genetically driven DNA methylation variation, transcription factors, histone post-translation modifications, chromatin remodelling and non-coding RNAs have all been shown to affect the molecular profile (including gene expression) of the cell and have the potential to alter its state and/or potential to differentiate, without affecting the DNA sequence itself (Bernstein, Meissner, and Lander 2007). The current challenge is to distinguish which are the key combinatorial regulatory patterns formed by these factors, which could be cause or consequence of disease.
As mentioned previously, Waddington referred to epigenetics from a developmental perspective. In a recent opinion article (Greally 2018), John Greally argued about the importance of clarity when using such terms as epigenetics, since its modern applications have significantly deviated from their original meaning (Haig 2004). There have been many misconceptions and misuses from borrowing terms from quantitative genetics to other fields of research (Deichmann 2020).
Lappalainen & Greally (Lappalainen and Greally 2017) built on the epigenetics landscape metaphor to propose a return to the use of a definition based on two types of cellular properties: “properties involving cell states (cellular reprogramming) and fates (polycreodism)”. Essentially, the cellular reprogramming epigenetic model includes reprogramming events at a cellular level, without altering the overall cell type (or DNA sequence). This is the most studied cellular model of epigenetic perturbations, where reprogramming in at least one cell type of unaffected individuals is compared to the same cell type in affected individuals. In contrast, the polycreodism model accounts for variation in proportions of cell types, formed all throughout development as a response to a perturbation, without necessarily affecting their molecular profile.
A word of caution…
Now you have a brief idea of the definition of epigenetics, and if you were paying attention you will notice how ambiguous it can be - even within the realm of science. It is possible that sometimes scientists embrace theoretical concepts from one field to another, without developing proper statistical methodology to test these assumptions. It is also possible this is in great part motivated by the publish or perish nature of life in academia…I am getting ahead of myself here, but the main point I am trying to to get to is this: ‘heritability’ in population genetics does not necessarily mean the same thing as ‘heritability’ in epigenetics, and this is an important distinction, because most of the analytical tools utilized in epidemiological studies of epigenetic variation in human populations have been adapted from quantitative genetics. We will touch on this subject later…
Glossary
- 💦 cytoplasm - a gelatinous liquid that fills the inside of a cell. It is composed of water, salts, and various organic molecules. The nucleus (and other cell organelles) is separated from the cytoplasm by a membrane.
- 🧬 DNA - deoxyribonucleic acid
- 🤰 embryogenesis - the formation and development of an embryo
- ☢️ somatic nuclei - (almost) every cell contains a nucleus, which serves both as the repository of genetic information and as the cell’s control center. Somatic nuclei, refers to nuclei from somatic cells, which are cells in the body other than sperm and egg cells (which are called germ cells). Humans somatic nuclei are diploid, meaning they contain two sets of chromosomes, one from each parent. Germ cells are haploid, meaning they contain one set of chromosomes.
- 🧫 totipotent - (of an immature or stem cell) capable of giving rise to any cell type or (of a blastomere) a complete embryo
References
Bernstein, Bradley E., Alexander Meissner, and Eric S. Lander. 2007. “The Mammalian Epigenome.” Cell 128 (4): 669–81. https://doi.org/10.1016/j.cell.2007.01.033.
Deichmann, Ute. 2020. “The Social Construction of the Social Epigenome and the Larger Biological Context.” Epigenetics & Chromatin 13 (1): 37. https://doi.org/10.1186/s13072-020-00360-w.
Greally, John M. 2018. “A User’s Guide to the Ambiguous Word ‘Epigenetics’.” Nature Reviews Molecular Cell Biology 19 (4): 207–8. https://doi.org/10.1038/nrm.2017.135.
Gurdon, J. B., T. R. Elsdale, and M. Fischberg. 1958. “Sexually Mature Individuals of Xenopus Laevis from the Transplantation of Single Somatic Nuclei.” Nature 182 (4627): 64–65. https://doi.org/10.1038/182064a0.
Haig, D. 2004. “The (Dual) Origin of Epigenetics.” Cold Spring Harbor Symposia on Quantitative Biology 69 (January): 67–70. https://doi.org/10.1101/sqb.2004.69.67.
Lappalainen, Tuuli, and John M. Greally. 2017. “Associating Cellular Epigenetic Models with Human Phenotypes.” Nature Reviews Genetics 18 (7): 441–51. https://doi.org/10.1038/nrg.2017.32.
Waddington, C. H. 2012. “The Epigenotype.” International Journal of Epidemiology 41 (1): 10–13. https://doi.org/10.1093/ije/dyr184.
———. 2014. The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Biology. 1st edition. Routledge.